In Figure 4, you provide evidence that ORF12 catalyzes the periplasmic transfer of α-Ribf via a GT-C fold mechanism, establishing it as the founding member of the GT136 family. The structure-guided mutagenesis and activity assays clearly identify key catalytic residues. However, one aspect that merits further clarification is the functional role of the conserved D31 residue, which you propose as the putative catalytic base.
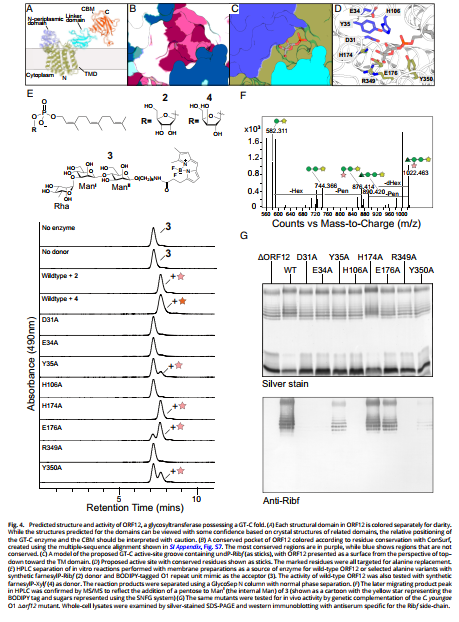
Given that many GT-C enzymes utilize a divalent cation or water-mediated network for catalysis, did you assess whether D31 coordinates a metal ion, or if its catalytic function is strictly reliant on direct proton abstraction? Additionally, was any structural or biochemical evidence (e.g., metal-dependence assays, pH profiling, or rescue by acidic residues) considered to validate its proposed mechanistic role?
Understanding whether D31 acts in a classical acid/base role or is structurally stabilizing could further substantiate the mechanistic model of GT136 enzymes and may offer broader insight into GT-C fold diversity.

