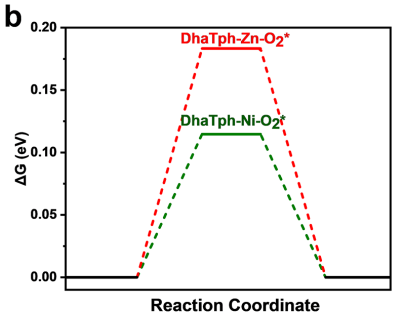
In Figure 4b, the free energy diagrams suggest that O₂ adsorption is significantly more favorable on DhaTph-Ni than on DhaTph-Zn, which is used to support the conclusion that Ni-containing COFs promote electron transfer and favor O2•- formation, while Zn-containing COFs facilitate singlet oxygen ( 1O2) generation. However, ¹O₂ formation typically relies on energy transfer mechanisms, which are generally more efficient under conditions of weaker O₂ binding. Given this, how do the authors reconcile the relatively weaker O₂ adsorption on DhaTph-Zn with its enhanced 1O2 yield? Wouldn’t the stronger O₂ binding in DhaTph-Ni intuitively favor longer-lived excited-state interactions needed for such transfer? Is there any spectroscopic or kinetic evidence provided to clarify whether O₂ adsorption strength alone is a reliable predictor for distinguishing between electron transfer and energy transfer pathways in this case?