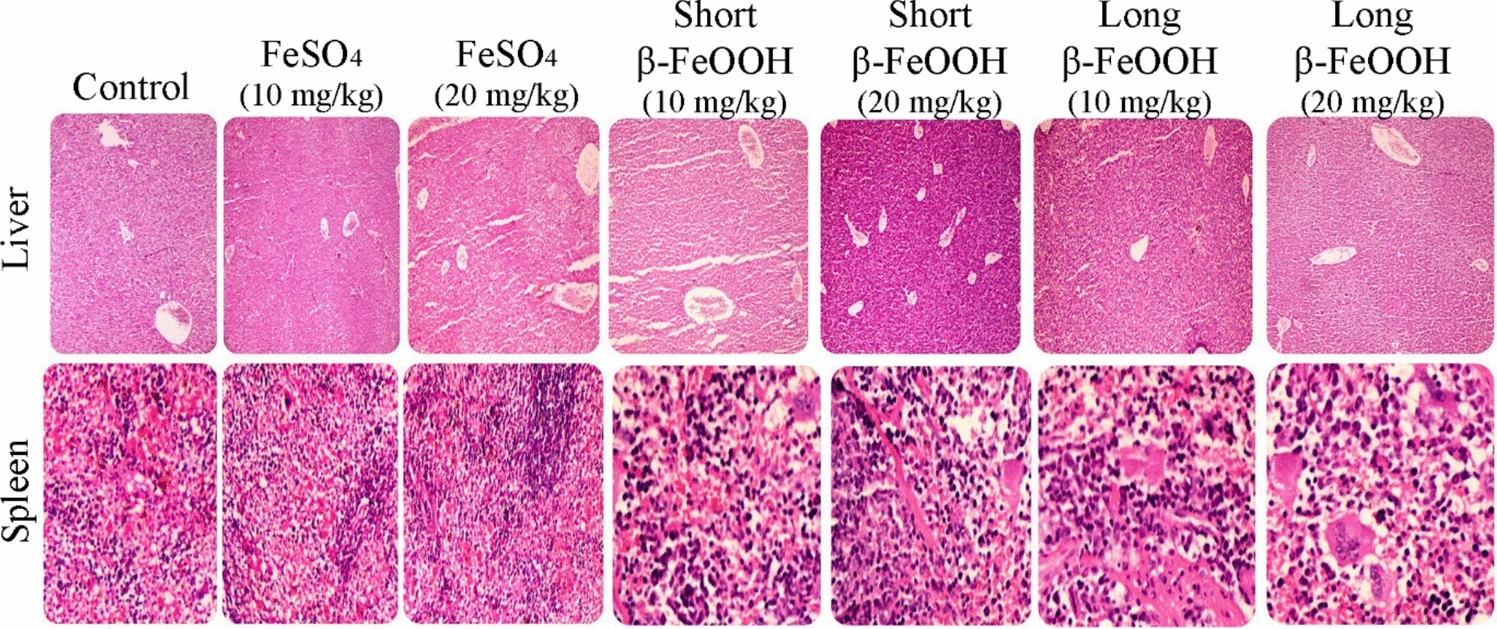
Figure 9 presents histological micrographs of liver and spleen tissues following one-month oral supplementation with various iron formulations, including β-FeOOH nanorods of different lengths and FeSO₄. The images are integral for assessing tissue-level safety and biocompatibility of the tested nanomaterials.
Notably, across all treatment groups, no significant pathological alterations were observed in hepatic or splenic architecture. The only minor deviation was the presence of several multinucleated giant cells, which may suggest a low-level immune or phagocytic response, possibly related to the particulate nature of nanorods. However, there was no evidence of inflammation, necrosis, fibrosis, or architectural disruption, as confirmed by both hematoxylin-eosin (H&E) and Masson’s trichrome staining.
These findings reinforce the in vivo biocompatibility of β-FeOOH nanorods, regardless of their length, and suggest minimal histopathological impact on major iron storage and detoxification organs at the tested dosages (10 and 20 mg/kg). Thus, Fig. 9 supports the study’s conclusion that short β-FeOOH nanorods are a safe and promising candidate for next-generation oral iron supplementation. However, future investigations incorporating ultrastructural or immunohistochemical analyses may better elucidate the origin and implications of the observed multinucleated cells.