The main concerns as posted for the preprint version of this study also apply to the published version. I will summarize them here again.
- It is unclear if ethical approval permits were obtained and if patients properly consented.
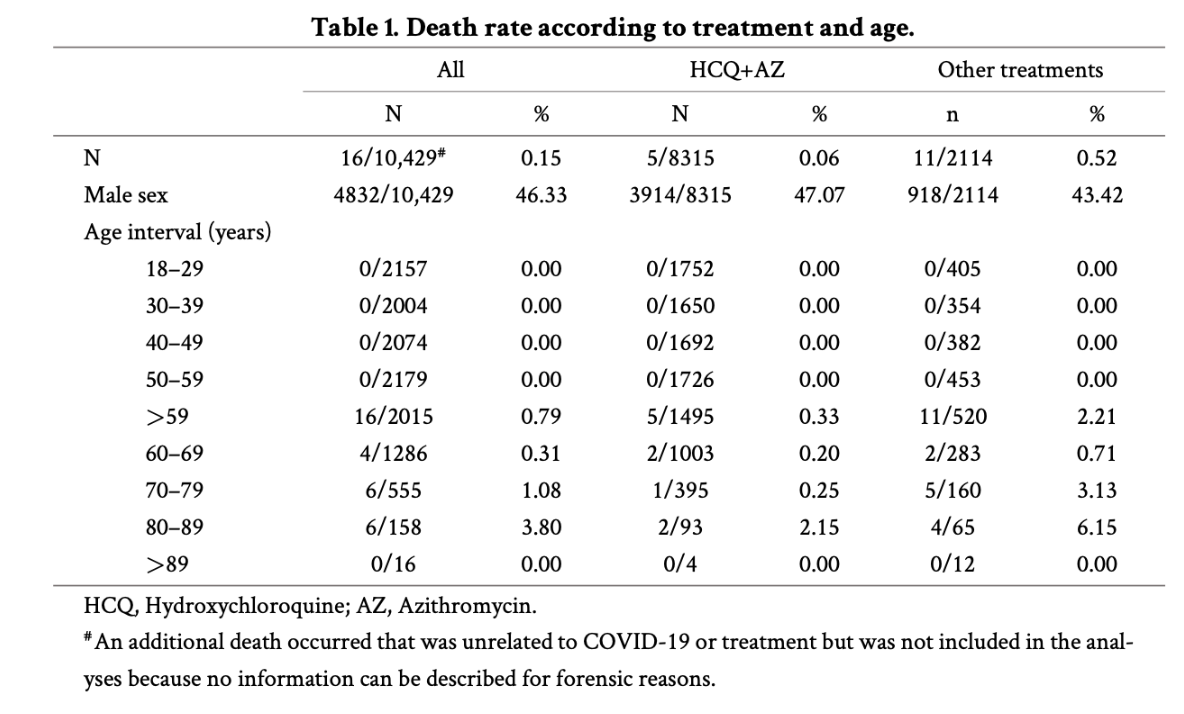
- The HCQ+AZ treatment group (n=8315) is compared to a heterogeneous ‘other’ control group (n=2114) consisting of patients who only received HCQ, only AZ or none of these two drugs. The treatment group was younger than the control group, while the control group appear to have had more comorbidities. Age and comorbidities are significant risk factors for COVID mortality, so these two factors alone already could explain why the treatment group did better than the control group.
- The age differences between the treatment and the heterogenous control group can be deduced from Table 1. From the numbers below it follows that the treatment group was younger than the control group. This is not a good starting position to be in when conducting a study like this. Ideally, you want your treatment and control groups to be as similar as possible before starting the study.
Older than 59 years:
HCQ+AZ treatment group: 1494/8315 = 17.9% was older than 59 years.
Other/control group: 520/2114 = 24.6% was older than 59 years.
Older than 70 years:
HCQ+AZ treatment group: 492/8315 = 5.9% was older than 70 years.
Other/control group: 237/2114 = 11.2% was older than 70 years.
Older than 80 years:
HCQ+AZ treatment group: 97/8315 = 1.2% was older than 80 years.
Other/control group: 77/2114 = 3.6% was older than 80 years.