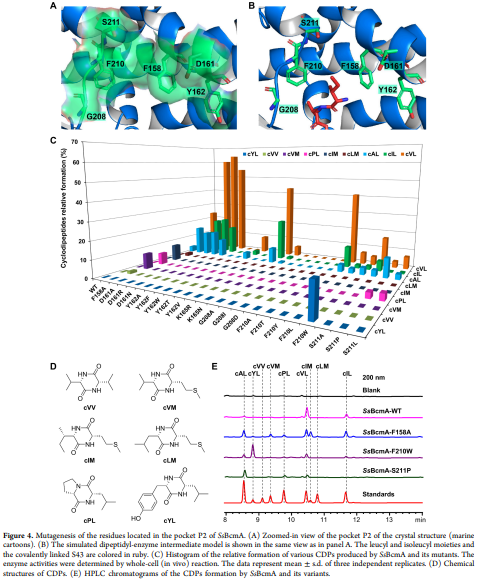
Table 1 presents crystallographic refinement statistics for SsBcmA, including completeness (91.1%) and redundancy (1.8). These values are lower than typically expected for high-resolution structures, raising concerns about the reliability of the electron density maps for certain regions, especially the partially resolved loop (residues 161–175). Could the authors provide clarification on how these limitations might affect the interpretation of active site interactions and substrate specificity?
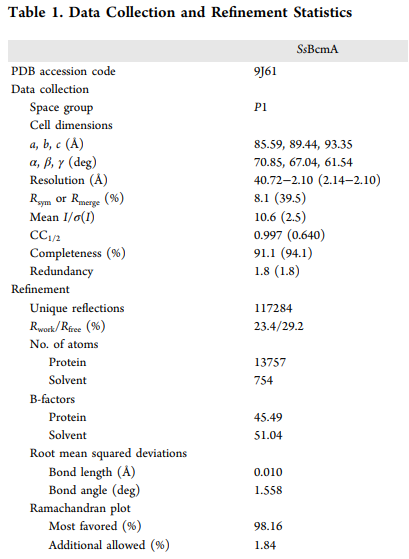
Additionally, Figure 4 shows shifts in product ratios upon mutagenesis but does not provide statistical measures of variability. Were multiple replicates performed to ensure the reproducibility and robustness of these findings?