In Table 5, the relative difference between predicted and in vivo AUC values for the 110 µg dose is reported as 10.4%, yet this deviation appears substantial when considering clinical relevance. Could the authors clarify whether sensitivity analyses were conducted to assess how variations in key model parameters (e.g., dissolution rate or mucociliary clearance velocity) influence AUC predictions? 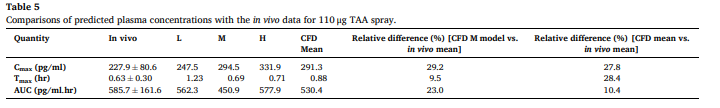


In Figures 10 and 11, although error bars indicate variability in in vivo data, the CFD model predictions for Cmax appear consistently close to the upper limits of these ranges. Could the authors clarify whether this reflects systematic biases in the model’s assumptions (e.g., nasal geometry or dissolution rates)? Additionally, how was the impact of intersubject variability incorporated into the predictions, especially for drug deposition and dissolution in different nasal regions? Providing more context on these aspects would enhance the interpretation of the model’s performance.